
Dr. Kaushik S. Bhojani, a Consultant Rheumatologist and the Head of Rheumatology Services at Fortis Hospitals, Mumbai Cluster, talked about the importance of clinical trials at the Sclerocon conference 2024 organized by Scleroderma India. He highlighted the process of clinical trials and how participating in the clinical trials can benefit patients and community.
Clinical trials play a crucial role in the progress of medical science. However, many individuals are hesitant to participate in a clinical trial due to fears related to experimentation, which can hinder the development of new treatments. Trials are done with an aim to understand the problem, testing medications and monitoring their effects. All these process are important for developing effective treatments. That is not proven by chance, it is done through extensive clinical research. For instance, in case of medications like mycophenolate and cyclophosphamide, mycophenolate is preferred due to its effectiveness and lower side effects. That is proven through extensive clinical trials. Indian patients can participate in the clinical trials that provide access to advanced treatments. This can provide valuable data for the global medical community. Doctors can understand how well treatments work for patients and this can help in improving future therapeutic options.
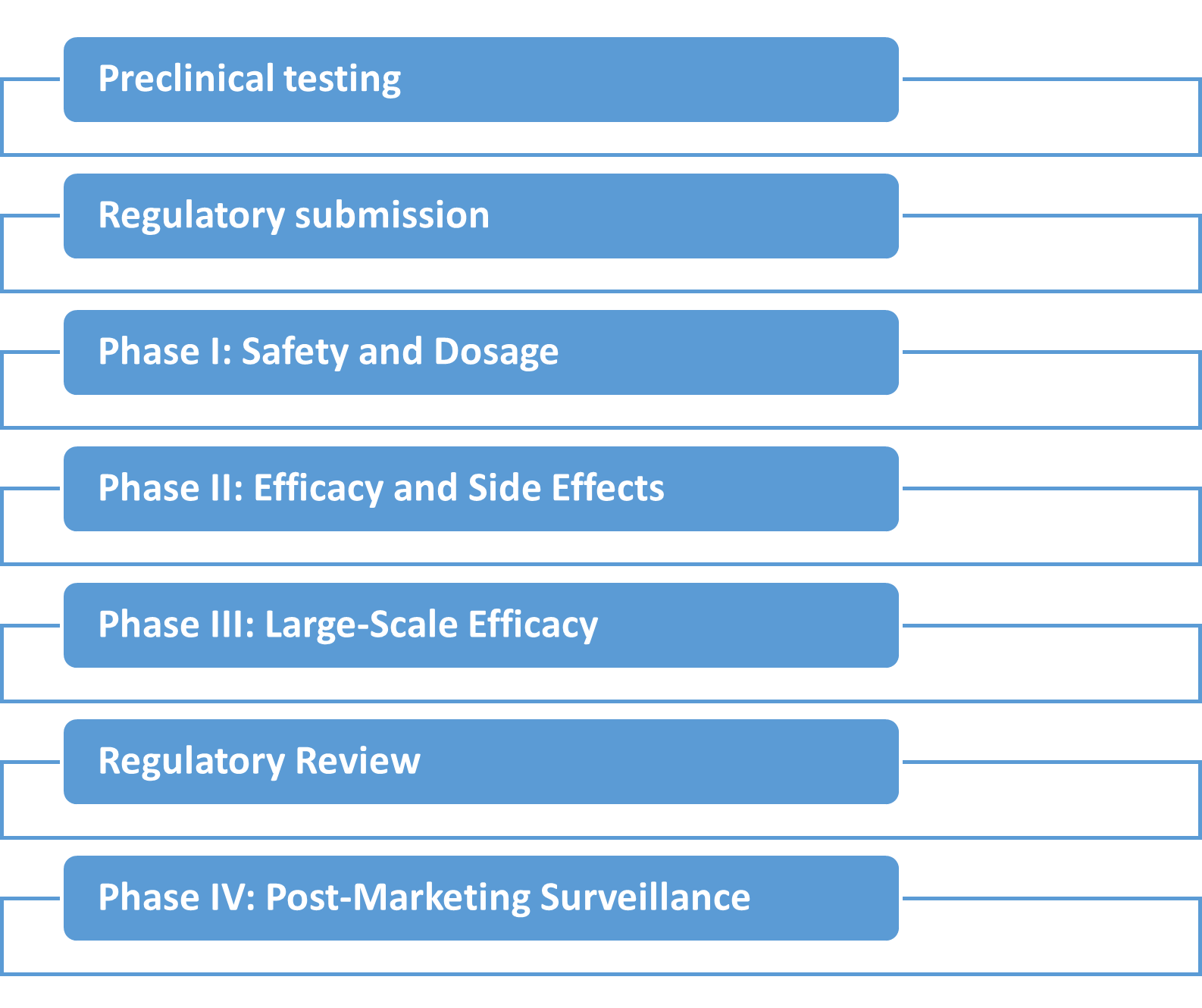
What is the process for a clinical trial?
- Preclinical research is done by testing the drugs in the laboratory. Researchers identify a chemical compound that could treat a specific medical condition. Its potential is evaluated with various tests and the chemical or cytokinetics is used in phase 2 clinical trials.
- In phase 2, the medication is administered in small amount to monitor its side effects. After that the dosages are gradually increased to study the drug safety and efficacy. Initially the drug is tested on animals, then a small group of humans.
- A larger patient group is enrolled for phase 3. Blinded trial is conducted, means neither the doctors nor patients are aware about the group they are in. Randomly patients are assigned to receive the medication or placebo.
- Regular blood tests, ECGs, CT scans, X-rays and other health checkups are done during each visit of the patients enrolled in clinical trials. Close monitoring is done and data is collected meticulously on the patients’ response to the drug. According to that the dose is adjusted.
- After the trial gets over, the data is unblended to check which patients received the medications and which patients received the placebo.
- Following to that an open label phase is performed and the patients who initially received a placebo will now receive the medication. This will help evaluate the drug effectiveness, safety, and side effects.
- In India, the FDA (Food and Drug Administration) approves the drug. So if the drug proves successful, it is submitted for licensing to FDA. The FDA reviews it and if satisfied, the drug is approved for the widespread use.
- If any drug is approved after clinical trial, then there is no animal testing required. For instance in past, studies on cyclophosphamide and mycophenolate, demonstrated the effectiveness and side effects of these drugs, leading to their current use. Followed by that newer drugs like rituximab and tocilizumab were tested, with tocilizumab being already approved worldwide.
- In India, clinical trials are very limited. However, with specialists’ efforts there is now a significant opportunity for Indian patients to participate in international trials and benefit from modern treatments also.
CONQUEST study –
It is a new approach where two drugs are tested simultaneously. Participation in these trials can provide insights into how well the drug works for Indian patients, considering different genetics and lifestyle factors. In one trial 100 patients receive the drug and in another, 100 patients receive placebo. This method ensures that a greater number of patients receive the medication and helps make the trial process more efficient and more effective. By participating in this trial, patients can access advanced treatments and contribute to valuable research. It also helps in creating more accurate data for Indian patients that can be helpful for better treatment options for scleroderma in the future.
Note : This is a loosely edited text of the presentation made by Dr. Kaushik Bhojani at the Sclerocon 2024 Conference - Importance of clinical trials
Dr. Kaushik Bhojani is a Consultant Rheumatologist and the Head of Rheumatology Services at Fortis Hospitals, Mumbai Cluster. With more than 20 years of experience in his field of specialization, he has deep expertise in treatment and management of all types of rheumatic diseases as well as auto immune diseases. He also has a special interest in the rehabilitation of patients with disabilities due to long standing arthritis.






