
In the UNPACKING podcast series, we start with a discussion on a new and exciting innovation in cancer treatment, the CAR -T Cell Therapy with Dr. Hari Menon. During the course of this conversation, we will unpack what CAR -T is, where it stands today. Is it really what you think it is and how we can take informed decisions on this new treatment?
Dr. Hari Menon is Professor of Hematology and Head Medical Oncology at St. John's National Academy of Health Sciences in Bangalore. He has been in the field of medical oncology for more than two decades, starting with his training at AIIMS in Delhi. He was faculty and professor of medical oncology at Tata Memorial Centre, Mumbai, wherein he focused in the specialized field of hematological malignancies and stem cell transplant. He has also been involved in CAR -T clinical trials. He's been a member of ICMR task force for guidelines for blood cancers. He is also a very active supporter of patient education, patient advocacy, and supports better outcomes for patients.
What is CAR -T cell therapy? What does it mean in lay language or in plain language?
CAR -T cell stands for chimeric antigen receptor CAR-T -cell, so antigen receptor T -cell. Basically, it's an engineered T -cell. In the human system, the T -cells are responsible for immune surveillance, especially against malignancies. T-cells target the abnormal tumour cells which it identifies through various mechanisms for destruction. Many cancers are amenable to this normal immune surveillance but some cancers. esp. blood cancers have incredible abilities to avoid the immune surveillance system, and that's how they tend to survive. In fact in 2017 Nobel Prize was awarded to identifying these mechanisms as to how these cancer cells survive.
CAR-T cell therapy is a type of treatment that helps the immune system help identify these difficult cancer cells, so they can be killed.
Every cancer cell has some antigen expressed on its surface. The T-cells of the body have receptors that are able to identify these antigens and attack the cancer cells and then destroy these cells through various mechanisms. But many times, the T-cells do not express these receptors and fail to attack the cancer cells. So, the idea of CAR-T cell is to make the T cell express these receptors called CAR-Through manipulation at the nuclear level in the lab. CAR is a synthetic receptor that is made to bind to a specific antigen on a cancer cell. The modified T-cells are now called CAR-T cells.
What is the process of CAR-T cell therapy?
Process: First extract the T cells from the patient, manufacture these T cells to express specific receptors and then give it back to the patient. The modified T-cells, called CAR-T cells will now recognise and attack to destroy the cancer cell.
Because the CAR-T cell is a clone of the normal T- cell it's called a chimera. Hence, the term chimeric antigen.
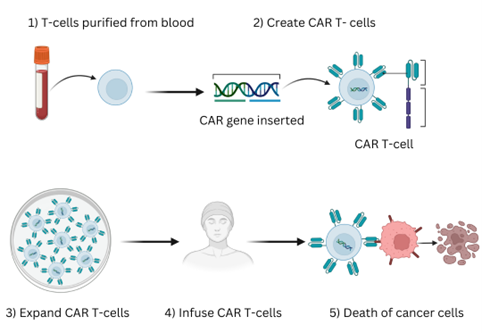
Figure 1: https://test2.origene.biz/research-areas/car-t-cell
How are CAR-T cells modified?
Essentially, T cells that are present in the patient are removed, harvested and then manipulated through vectors to introduce certain codes into the DNA sequence, which will allow for the expression of these. These codes are responsible at the genetic level to express on the surface certain receptors. And once that code is inserted into the DNA, it naturally expresses that. Then comes the point of expanding the T cells in the laboratory. When you expand all those, not everything is incorporated with the receptors. So you have a percentage of those. Then you know the viability of the cells and give it back to the patient. Once inside the patient, the immune mechanism takes over and again it expands within the patient's body and attacks the tumour.
People dealing with cancer now understand terms like targeted therapy, chemotherapy, immunotherapy. What type of therapy is CAR-T?
By definition, it is a version of immunotherapy because you stimulate the immune system to do the job that it is supposed to do. Since these are live cells that are manipulated, we would rather call this as cellular therapy rather than refer to it as immunotherapy. It would fall into the same bracket as allogenic stem cell transplants, where donor cells are introduced to the patient to boost the immunity. In a way, CAR-T is a type of transplant, only that because it is a patient's own cell, it would be categorized like an autologous transplant. That's a patient's own cells are being used.
Is CAR -T cell therapy one time treatment ?
If the CAR-T is targeting one particular antigen, it is a one -time treatment. In lymphomas, only the CD19 antigen has been identified for target. CD19 is a protein found on the surface of most B-cell cancers. So currently, when we're talking about CAR-T cells, T-cells are modified in the lab to express a CAR-That can recognize and bind to CD19. This can be done for patients with B-cell lymphomas or B-cell leukemias.
In the future, we might have other antigens that we want to target, and then you can use a different type of CAR for that antigen. Nowadays, we are also developing CAR-Ts for dual antigens. It targets two different antigens on the surface of the cancer cell. Another type of CAR-T is the bispecific antibody therapy, which has 2 functions. One limb actually targets the normal T cells, and the other limb targets the antigens on the tumour cells. So in effect, it brings the T cells and the tumour cell in close proximity so that the T cells are able to identify and kill the tumour cell. Such CAR-Ts treatment which uses bispecific antibodies are actually multiple treatments. So it's not a single treatment but will be given like chemotherapy in multiple cycles.
Are there multiple products for CAR -T?
You now start to hear different brand names even within India. They are basically different protocols that are used to engineer the T-cell, but the target remains the same. The vector used may be a little different or the way it attaches to the linkers may be different. There are many aspects to the structure of the CAR-T cell and various modifications in the way that the structure is formed. That's what makes it different, like different drugs or different pills. At the end of the day, the target remains the same.
When it comes to treating for lymphomas, the target is the CD -19. Some labs are looking at CD -22. In myeloma, the target antigen is known as the B cell membrane antigen or the BCMA.
Now we have B cell leukemia and B cell lymphoma are both with CD19 as the approved. Are those the only two categories of patients that are eligible for this therapy?
Patients with any B -cell lymphomas expressing CD -19 and have had two lapses in terms of standard treatment are eligible for CAR -T in India. Right now CAR-T is used only for such relapsed or refractory tumours.
Trials are going on for its upfront use but B-cell lymphoma with standard treatment have 50 to 60 % long -term cure rates. For a tumour that has almost a 60 % primary cure rate, we may not want to use it as upfront. There are ways of trying to recognize tumours that are at higher risk for relapses. Studies are ongoing for its upfront use.
What is Refractory Disease?
Refractory can be either primary or secondary. In primary refractory, the cancer has not responded to the primary treatment offered such as chemo or immunotherapy. Secondary refractory is when the cancer initially responded to treatment but then stopped responding and relapsed.
Relapse can be early or delayed depending on the time between remission and recurrence of the cancer. Early relapse is often associated with a worse prognosis than late relapse. Late relapses generally have a better prognosis than primary refractory or early relapsed cancers because they maybe more amenable to treatment.
Is CAR-T available for Multiple Myeloma treatment yet?
No, it is internationally but not in India. We have finished the phase two study and are awaiting
the results. We will then submit the results to the DCGI to for approval. Abroad, there are three different products that are available currently for use against myeloma which targets the BCMA antigen.
ImmunoACT is also conducting phase 2 study for another CAR for the BCMA antigen.
How many patients do you know are undergoing CAR -T treatment in India?
If patients have to undergo a CAR -T cell outside that of a clinical trial in India, they have to go abroad to the United States, Europe or China. There are a lot of exclusive centers in China which are offering routine CAR -T cell therapy. However, the costs abroad are significantly higher.
Currently, outside of a clinical trial, it's not available in India. We have 24 patients who have been part of our multi -centric study. We project that the cost in India would be less than 1/3th or 1/4th the cost abroad.
What is the success rate or the outcomes of CAR -T?
With our own CAR-T over here in India, we have had fairly good success, especially for the B-cell lymphomas which is the CAR manufactured by ImmunoACT, the group which is a collaboration with the Tata Memorial Center and IIT, Mumbai. They have had good success rates, which is keeping in with the international data, but the data is still maturing in terms of long-term outcomes.
The results have been probably a little better with the lymphatic leukemia group of patients and not as good for the B cell lymphomas. But we are talking about patients who are very refractory to their disease and it is more or less comparable with the data from the international database on CAR -T cells for these indications.
The data for myeloma is very promising. The first patient has completed more than one and a half years post start of CAR-T treatment. It looks promising, but I think we should never talk on all the phase 1 data until we have a larger cohort over there. But in terms of efficacy, I think it's very good.
People kind of always look at a new therapy with a lot of hope, is CAR-T a guaranteed cure? Is it too expensive an option, what should patients be looking at when considering it?
When we talk about CAR-T cells, there are a lot of knowns and unknowns over here. A lot depends upon how the immune system is going to work and respond. A lot of other factors matter for example the micro environment of the tumour or the immune competence of the patient, extent of the disease etc. Any cellular therapy whether it is an allogeneic stem cell transplant or CAR-T therapy or targeted therapy, work better when the volume of the disease is low. When you have a very high-volume disease, it is unlikely that patient will respond well because there are a lot of other factors that come into play. Having said that, we go with the understanding that this is one of the ways to treat B cell lymphomas, especially with refractory disease but there is no guarantee that it will work. More importantly, whether the response can be sustained over the period of time.
There is something called the persistence of the CARs in the system to continue to do the work that it has to do. There is a great ability for the tumour cells to escape the immune response and sometimes there is a fatiguing of the immune response which will allow for the tumour to grow. It also depends upon how aggressive and fast-growing the tumour is.
What is the data that we have so far on CAR -T in terms of how long does that persistence of the CAR last? And for what percentage of patients?
If you look at the global data, the responses can range between 40 to 80 percent, but that's not always translating into benefits or the persistence. Long -term outcomes are somewhere between 50 to 60 percent for all CAR -T cells. As of now, our data is still not fully mature but the long-term follow-up from the Indian CAR -T for CD19 is in excess of 40 %. It cannot be justified for us to commit on to that data till after a longer follow-up on these patients.
For the Indian CAR-T, how you know, what is the oldest or the longest it has persisted?
It was more than two years now. We can never predict as to who's going to respond. We can just hope. The primary thing is to get a response and then to see how well they do. The adage is that if you have a response, then it is likely that patient will go on to sustain that response.
Is there a mechanism to assess whether a patient is eligible such as an assessment?
In chemotherapy, we expect patients to have varying responses in terms of tolerance to the treatment. That doesn't hold true for CAR-T. Even elderly people above the age of 80 can undergo CAR-T therapy because there is less toxicity that is induced unlike chemotherapy. Practically any
patient is eligible for a CAR-T cell option for treatment, provided they are eligible.
Sometimes the kind of chemotherapy the patient has received earlier may impact the ability to manufacture the CAR -T cell.
Do specific chemotherapy protocols make a difference for eligibility for CAR-T?
Yeah, it does make a difference especially in low -grade lymphomas, which receive a certain kind of chemodrug called Bendomestine. Studies have shown that when patients receive Bendomestine, it becomes difficult to harvest and manufacture T cells over there. For such patients, we wait at least for a minimum of six months before we attempt to harvest the CAR-T cells.
Unfortunately six months maybe too long for a patient's survival itself in the presence of an aggressive disease.
Are CAR -T trials happening for pediatric ALL, acute lymphoblastic leukemia?
Yes, trials are on at Tata Memorial; one for lymphomas and another was for acute lymphatic leukemia which is more common in the pediatric age group.
Are there any side effects or complications that patients should expect?
There are a lot of problems, unique problems that develop like any other allogenic stem cell transplant. Whenever we are trying to manipulate the immune system, we might think that we can control it, but we can never control the immune system. What we know is only the tip of the iceberg, as far as the way the immune system works.
Cancer patients are compromised by virtue of the disease that they have and the multiple treatments they have received before. Over and above that, we are trying to induce an immune -mediated response, which will also cause a lot of suppression. Normal B cells also express CD19, so during CAR-T, there might be destruction of many of the normal B cells and lymphopenia develops. The function of B cells is to secrete immunoglobulins that protect us from various infections, especially viral infections.
In order to start CAR-T -cell therapy, the body has to be prepared by creating an environment which suppresses all the B cells as much as possible. We do this by giving chemotherapy, which causes some amount of immunosuppression in the first one to 30 days. Then when the CAR-T cells are introduced into the patient's body, it stimulates an immune response, which will secrete a lot of cytokines, etc., which in itself will induce an immune suppression in the body. Later on, because of the prolonged suppression of the B cells, you get a reduced amount of globulins in the body, which makes you prone to infection. So over a period of time, at various levels, you have a sustained immune suppression, which makes you prone to infections. That is where the challenge lies in treating with CAR-T therapy. We have to artificially give IV immunoglobulins to the patient to sustain the levels for some degree of passive immunity so that they don't develop infection. While it's an effective therapy, the challenges are actually how you can handle problems like cytopenia that is low blood counts, low immune system, low immune responses and increased risk for infections. Cytopenias may continue to remain lifelong for the patient.
Sometimes you can also have immune mediated immune effector neurological symptoms in patients. This is particularly true in myeloma CAR-T therapy because certain targets affect the basal ganglia cells which also expresses the BCMA antigen.
There are various side-effects that can develop but we still go ahead with it since you cannot predict who is going to develop the side-effect and who is not. Sometimes the patient might get treated and cured of the disease, but end ups having to manage all the infections for a long period of time.
In terms of the neurological side-effects, do patients end up getting behaviour issues?
Yes one of the manifestations is known as ICANs or Immune effector cell-associated neurotoxicity syndrome which causes neurological and psychiatric symptoms. We always monitor these patients. Usually this happens not in the early phase but in the second week or so. It's important to recognize this and then manage with steroids. Again, these are not predictable. It can happen to anyone.
For ICAN, are patients expected to go to a psychiatrist for treatment?
No, because this is immune mediated, the front line is to start steroids in these patients. If ICANs is due to cytokines or the tumor, antibodies like Siltuximab or Tocilizumab are used.
Can ICAN be compared to chemo -brain?
Chemo-brain is something that one develops long after the chemotherapy. It's not the same. It is more of a confusional state that happens in ICANs and in the chemo-brain is more of a broad kind of neuro response. ICANs is an immune -mediated response while chemo-brain is not.
Post CAR-T, do patients need to stay in an immune-safe environment and for how long?
That is unfortunately, not predictable.
In allogeneic stem cell transplant you ablate the bone marrow, so the patient is a sitting duck for about 15 to 20 days for infections. In CAR-T, we give a milder chemotherapy at the start called a lymphoid depletion therapy. We are a little careful for the first 10 to 15 days, and if the patient has not had too much of a problem, then they're back to regular life. We recommend precautions like mask wearing, avoiding infections as much as possible, not getting exposed to extreme weather etc, but we don't recommend total isolation. In a transplant, we impose some isolation for the first three to four weeks until the graft gets in and starts functioning. We don't do that with CAR-T therapy. The problem in CAR-T cells is the sustained susceptibility to infection over a period of time. But that can be variable for different patients, so we don't ask for strict isolation.
Do patients need to redo all of their vaccinations ?
Yes, that is required. We are not modifying their entire immune system. If the patient has not been vaccinated earlier, we do advise for regular vaccination for influenza and pneumococcal infections. That is usually done about maybe around six months to one year after therapy. There are various schedules for that. For any vaccine, you should be able to mount a response, like an antibody-based responses. In CAR-T since B cells are depleted, which are responsible to generate those immunoglobulins, you may not get the kind of response that you desire from a vaccination schedule in these patients.
What are the common causes of mortality, post -treatment? Is it just that the treatment didn't sustain or is it that there were infections?
The two main causes are disease progression and infections. Sometimes immediately post-infusion of the CAR -T cells, patient can get acute reactions like cytokine release. This is a big inflammatory response that is created, which causes a lot of hemodynamic compromise, hypotension, capillary leak, and pulmonary edema which can be quite refractory. Those are life threatening. These are the acute causes for morbidity and mortality associated with CAR-T cells. Delayed causes of morbidities include infections, and cytopenias.
What is the cost of CAR -T in India?
Since it's a very complicated and stringent manufacturing process, much of the cost goes into the
manufacturing and the vector used. Viral vectors are expensive and patented. The lab requirements are quite stringent because live cells are used and they must maintain their integrity. A lot of reagents are used and the environment within the lab needed to produce viable cells is an expense.
On an average in the West, it's around 250,000 US dollars. With additional cost for the supportive care that is required, it can be close to around $400 ,000 to $500 ,000. Whereas in India, we are talking in terms of about anywhere between 35 to 40 lakhs for the ImmunoACT ones. That cost is going to come down over a period of time, probably to the tune of about 20 to 25 lakhs. Immuneel, which is also manufacturing the CD19 CAR-T costs around 40 lakhs.
Beyond the manufacturing cost, you have to give allowance for the supportive care that is required. The cost for immunoglobulins, steroids, antibiotics, blood products support like platelet transfusions etc all add up.
With all this immune activity and compromise, could patients end up with autoimmune conditions?
A lot of autoimmune issues can manifest like lung toxicity, renal toxicity, or liver toxicity. There have been some concerns about CAR-T cells in the future because the fear is if the viral vector itself can
become oncogenic and induced. There have been reports of unusual T cell lymphomas where the T cells given as part of the CAR -T cell therapy may have become malignant and this has been identified because the viral vectors in those malignant clones have been identified. But these have been case reports that have developed and I think the data has to mature for that.
In the team of doctors that will support a patient who undergoes CAR-T, is it just the oncologist?
It would be a good idea to have an immunologist on board but generally the hemato-oncologists who are dealing with transplants on a routine basis will be able to manage the issues that develop. It's more important to recognize problems even before starting the treatment. The faster you recognize a problem and initiate a treatment, the better are the results. Experience of the doctor will really matter. For example, if a patient develops ICANS, you don't call the neurologist, but need to have the treating physician understand what the problem is and take a measure. You don't need the neurologist to tell you that this is ICANS.
As of now, oncologists manage all these issues, but as the number of patients increase and given that patients are traveling long distances etc. it will help to have immunologists on board. And often in an Indian context where we are not very referral driven, patients might end up in wrong hands, so educating the patients is very important.
Currently, how many centres in India are offering CAR-T?
There are several centres, but the manufacturing is done only by a few like ImmunoACT and Immuneel and maybe in the future, Aurigene upon approval. There are several other companies which are looking at setting up manufacturing facilities for CAR-T cells in India.
In the next three or five years, a lot more companies will be coming forward offering CAR-T cells.
In fact, we will be embarking on to trial where we call off the shelved CAR-T cells or allogenic CAR-T cells. This is a different way of manufacturing CAR-T cells, where we have a healthy donor to provide the T cells. It is akin to doing an allogenic stem cell transplant where you are using a healthy donor's T cells and manipulating them for acceptance. It's not like HLA matching but we manipulate the CAR-T cells to knock off certain receptors on the cells via CRISPR technology such that it becomes accepting to the recipient. These are called off-the-shelf CAR -T cells and that is something that we might see in the future.
Once a patient decides to undergo CAR-T, what is the waiting time?
One of the issues with the CAR -T cell therapy is the manufacturing time. So, if I were to plan a CAR -T cell for a patient, I have to plan 6-8 weeks in advance so that the patient can be planned for harvesting followed by the manufacturing process of two to three weeks. This is the average time, but can be more. Sometimes the patient may not last the waiting time. So that's one of the deficiencies.
What are bi-specific antibodies vs CAR-T?
Bi-specific antibodies are a type of immunotherapy that can be used off-the-shelf meaning they are readily available for treatment unlike CAR-T which requires customised manufacturing. Bispecific antibodies have two binding sites, one that targets a specific antigen on cancer cells and another that engages immune cells (like T cells) to attack the cancer. The data says that they are almost as effective as CAR -T cells on a patient-to-patient basis but is very expensive and needs to be given frequently like chemotherapy at regular intervals. These are still in phase one study.
Do you see CAR -T as a stand alone therapy or a complementary therapy along with bone marrow transplant?
In acute lymphatic leukemia, many of the patients in our study underwent an allogenic stem cell transplant post CAR -T cell. You would look at that as a bridge to therapy, bridge to a transplant. But we rarely do allogenic stem cell transplants in lymphomas, it's almost exceptional. Yes, you may combine an allogenic stem cell transplant with CAR-T therapy in lymphatic leukemia, but that may not be the case always because you have long-term results for acute lymphatic leukemia, even with the CAR -T cell therapy alone. That's an evolving thing as far as CAR -T cell is concerned.
Can CAR -T be developed for daycare administration?
You can give the infusion in a day care setting , since it just takes 15 minutes to give the infusion of a manufactured cell. But we have to monitor patient for side-effects within the first few days which develop suddenly. Hence, we prefer to admit the patient for at least a week post therapy. Sometimes patients end up with a hemodynamic compromise with hypotension and will require intensive care support. In our phase one study, all of our patients were at the ICU because we needed to know how to be ready for any side-effects. So as of now, even for the first few cycles of bispecific antibody therapy, we admit the patient.
What question should the patient be asking the doctor or you know what are the answers they should get before they decide on going for CAR -T?
- Have all other options of treatment been exhausted?
- Am I refractory to chemotherapy?
- Am I a candidate for CAR-T? Is my Lymphoma or leukemia eligible?
- Is my current health status okay to undergo CAR-T?
- How can I prepare for CAR-T?
- Do I have enough time to wait for CAR-T (harvesting, manufacturing and treatment)?
- Is my tumour galloping? Will immune mediated therapies like a CAR -T cell therapy likely to work?
What are the CAR -T trials that are currently ongoing?
Currently, the clinical trial for Myeloma is closed for accrual. ImmunoAct may expand its Myeloma trial in phase 2 to more centers.
The CD19 car is currently available at multiple centres in the country from either ImmunoACT or Immuneel.
Immuneel is also researching the BCMA CAR-not sure whether the clinical trials have started.
One of our centers is embarking on to an allogenic CAR-T cell trial, which is the first in humans actually. We've just submitted our project to the IRB for approval. Once that comes in, we anticipate that later part of 2025, the phase one study may be launched.
This is an edited version of the podcast interview below. This has been edited for clarity and ease of understanding.















